Nevonen, D. E., Schaffner, J., Hanrahan, P., Shepit, M., van Lierop, J., Blank, D. A., & Nemykin, V. N. Journal of Porphyrins and Phthalocyanines, 2023.
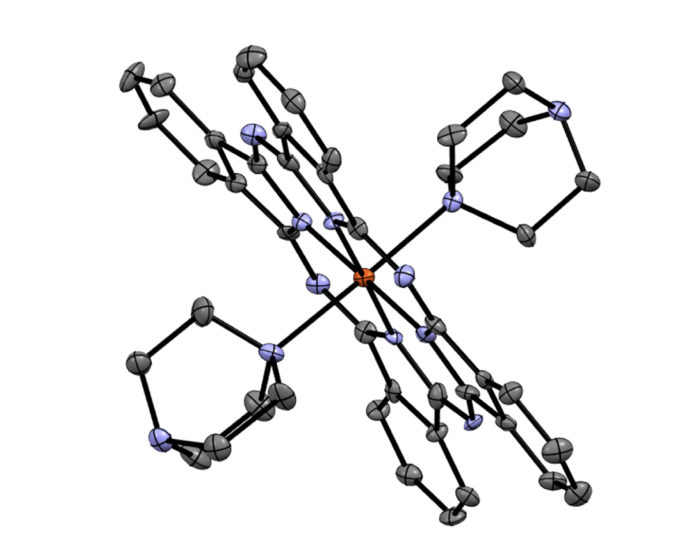
The elusive PcFe(DABCO)2 (Pc = phthalocyaninato(2-) ligand; DABCO = 1,4-diazabicyclo[2.2.2]octane) complex was prepared and characterized by UV-Vis, MCD, 1H NMR, and Mössbauer spectroscopies. The X-ray crystal structure of this complex indicates the longest Fe-N(DABCO) bond distance among all known PcFeL2complexes with nitrogen donors as the axial ligands. The target compound is only stable in the presence of large access of the axial ligand and rapidly converts into the (PcFe)2O 𝜇μ-oxo dimer even at a modest temperature. The electronic structure of the PcFe(DABCO)2 complex was elucidated by DFT and TDDFT methods. The DFT calculations predicted a very small singlet-triplet gap in this compound. The femtosecond transient absorption spectroscopy is indicative of extremely fast (∼∼200 fs) deactivation of the first excited state in PcFe(DABCO)2 with a lack of formation of the long-lived low-energy triplet state.