J. T. Moore, M. J. Dorantes, Z. Pengmei, T. M. Schwartz, J. Schaffner, S. L. Apps, C. A. Gaggioli, U. Das, L. Gagliardi, D. A. Blank, C. C. Lu, Angew. Chem. Int. Ed. 2022.
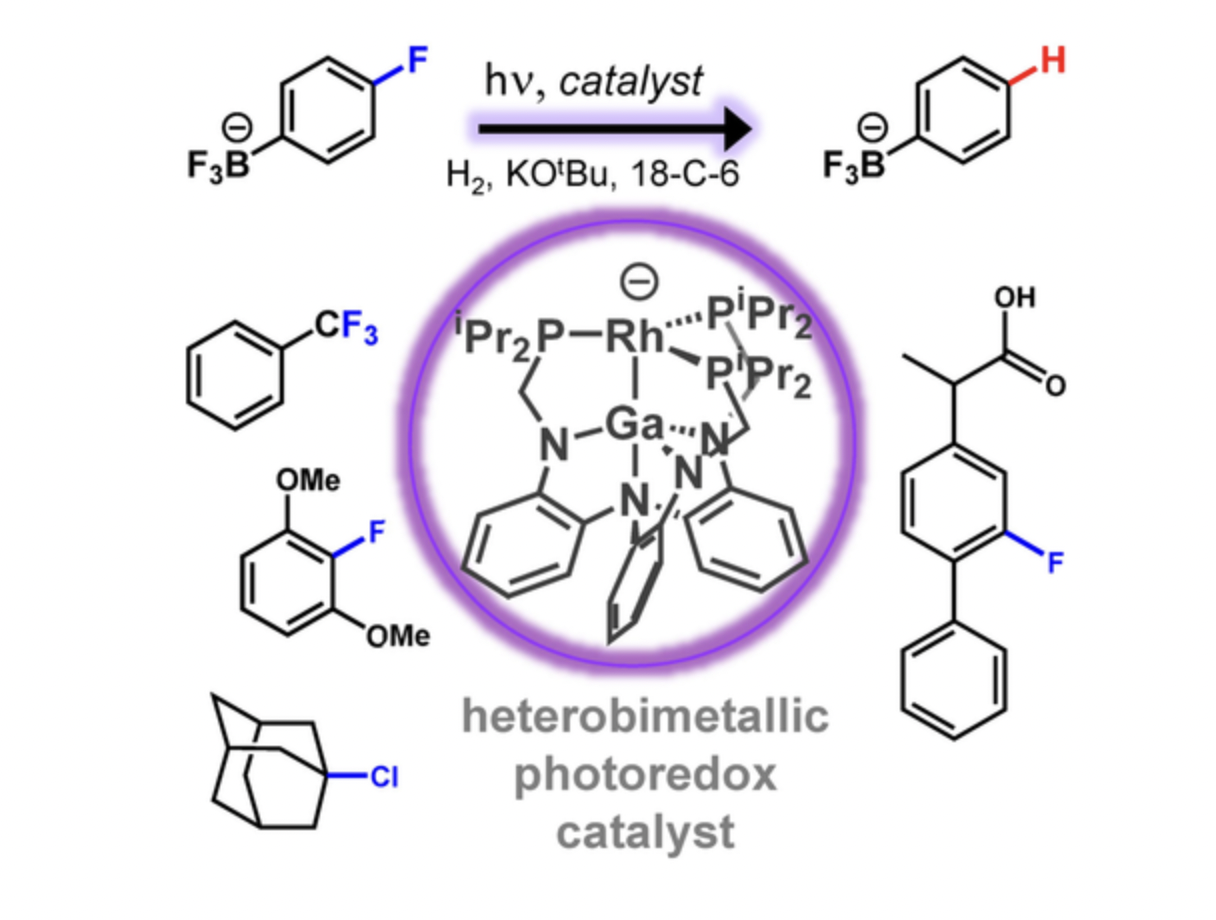
An anionic Rh−Ga complex catalyzed the hydrodefluorination of challenging C−F bonds in electron-rich aryl fluorides and trifluoromethylarenes when irradiated with violet light in the presence of H2, a stoichiometric alkoxide base, and a crown-ether additive. Based on theoretical calculations, the lowest unoccupied molecular orbital (LUMO), which is delocalized across both the Rh and Ga atoms, becomes singly occupied upon excitation, thereby poising the Rh−Ga complex for photoinduced single-electron transfer (SET). Stoichiometric and control reactions support that the C−F activation is mediated by the excited anionic Rh−Ga complex. After SET, the proposed neutral Rh0 intermediate was detected by EPR spectroscopy, which matched the spectrum of an independently synthesized sample. Deuterium-labeling studies corroborate the generation of aryl radicals during catalysis and their subsequent hydrogen-atom abstraction from the THF solvent to generate the hydrodefluorinated arene products. Altogether, the combined experimental and theoretical data support an unconventional bimetallic excitation that achieves the activation of strong C−F bonds and uses H2 and base as the terminal reductant.