Citation
Hanna M. Rhoda, Mathew P. Kayser, Yefeng Wang, Alexander Y. Nazarenko, Rodion V. Belosludov, Paul Kiprof, David A. Blank, and Victor N. Nemykin. Inorganic Chemistry, 2016, 55, 9549–9563.
Abstract
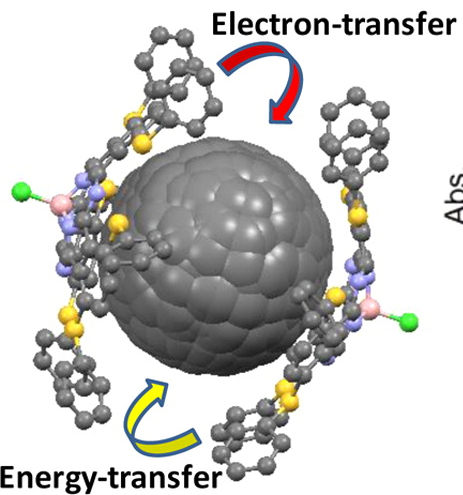
Noncovalent π–π interactions between chloroboron subphthalocyanine (1), 2,3-subnaphthalocyanine (3), 1,4,8,11,15,18-(hexathiophenyl)subphthalocyanine (4), or 4-tert-butylphenoxyboron subphthalocyanine (2) with C60 and C70 fullerenes were studied by UV–vis and steady-state fluorescence spectroscopy, as well as mass (APCI, ESI, and CSI) spectrometry. Mass spectrometry experiments were suggestive of relatively weak interaction energies between compounds 1–4 and fullerenes. The formation of a new weak charge-transfer band in the NIR region was observed in solution only for subphthalocyanine 4 when titrated with C60 and C70 fullerenes. Molecular structures of the subphthalocyanines 2 and 4 as well as cocrystallite of 4 with C60 fullerene (4···C60) were studied using X-ray crystallography. One of the C60 fullerenes in the crystal structure of 4···C60 was found in the concave region between two subphthalocyanine cores, while the other three fullerenes are aligned above individual isoindole fragments of the aromatic subphthalocyanine. The excited-state dynamics in noncovalent assemblies were studied by transient absorption spectroscopy. The time-resolved photophysics data suggest that only electron-rich subphthalocyanine 4 can facilitate an electron-transfer to C60 or C70 fullerenes, while no electron-transfer from the photoexcited receptors 1–3 to fullerenes was observed in UV–vis and transient spectroscopy experiments. DFT calculations using the CAM-B3LYP exchange-correlation functional and the 6-31+G(d) basis set allowed an estimation of interaction energies for the noncovalent 1:1 and 1:2 (fullerene:subphthalocyanine) complexes. Theoretical data suggest that the weak (∼3.5–10.5 kcal/mol) van der Waals-type interaction energies tend to increase with an increase of the electron density at the subphthalocyanine core with compound 4 being the best platform for noncovalent interactions with fullerenes. DFT calculations also indicate that 1:2 (fullerene:subphthalocyanine) noncovalent complexes are more stable than the corresponding 1:1 assemblies.
Publication URL
Image
Publication Title
"Tuning up an electronic structure of the subphthalocyanine derivatives toward electron-transfer process in non-covalent complexes with C60 and C70 fullerenes: Experimental and theoretical studies."
Publication Date