Citation
Yuriy V. Zatsikha, Natalia O. Didukh, Tanner Blesener, Mathew P. Kayser, Yuriy P. Kovtun, David A. Blank, and Victor N. Nemykin. Eur. J. Inorg. Chem., 2017, , 318–324.
Abstract
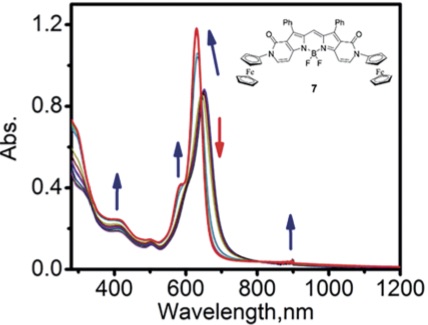
Mono- and di-(N-ferrocenyl-2-pyridone)-containing BODIPYs were prepared using a set of ferrocene-enamine cyclization reactions. The target compounds were characterized by UV-Vis, NMR, and mass spectrometry, while their redox proper- ties were probed by the electrochemical, spectroelectrochemical, and chemical oxidation methods. Redox properties of the di-(ferrocenyl)-containing BODIPY are suggestive of a lack of electronic coupling between the two ferrocene groups. Oxidation of the ferrocene(s) in the target BODIPYs results in their observable fluorescence, while transient absorption spectroscopy data indicate fast electron-transfer from ferrocene donor to the photoexcited BODIPY acceptor. Density functional theory (DFT) and time-dependent DFT (TDDFT) methods were used to elucidate electronic structures of the organometallic BODIPYs and confirm the presence of the low-energy metal-to-ligand charge-transfer states in the NIR region.
Publication URL
Image
Publication Title
"Preparation, Characterization, Redox, and Photoinduced Electron-Transfer Properties of the NIR-Absorbing N-Ferrocenyl- 2-pyridone BODIPYs"
Publication Date