Citation
Knudtzon, M.N.; Blank, D.A. Journal of Physical Chemistry B. 2020.
Abstract
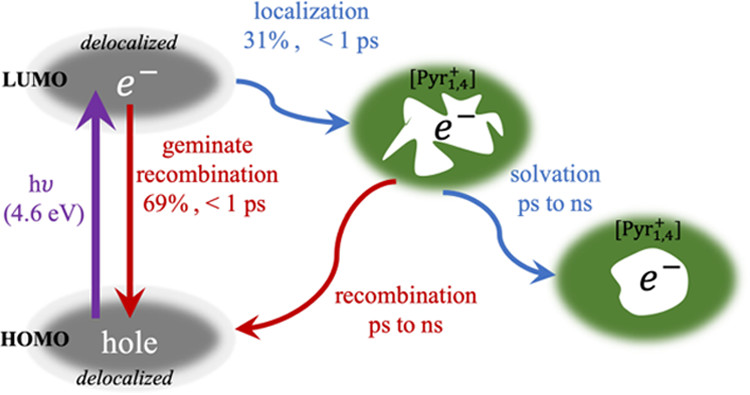
The ultrafast transient absorption spectrum of 1-butyl-1-methyl-pyrrolidinium dicyanamide, [Pyr1,4+][DCA–], was measured in the visible and near-infrared (IR) spectral regions. Excitation of the liquid at 4.6 eV created initially delocalized and highly reactive electrons that either geminately recombined (69%) or localized onto a cavity with a time constant of ∼300 fs. Electron localization was reflected in the evolution of the TA spectrum and the time-dependent loss of reactivity with a dichloromethane quencher. The delocalized initial state and spectrum of the free electrons were consistent with computational predictions by Xu and Margulis [ J. Phys. Chem. B, 2015, 119, 532−542] on excess electrons in [Pyr1,4+][DCA–]. The computational study considered two possible localization mechanisms for excess electrons, localization on ions, and localization on cavities. In the case of photogenerated electron–hole pairs, the results presented here demonstrate localization to cavities as the dominant channel. Following localization onto a cavity, the free electrons underwent solvation and loss of reactivity with the quencher with rates that slowed in time. The dynamics were similar to an analogous prior study on the related liquid [Pyr1,x+][NTf2–]. One significant difference was the larger yield of free electrons from photoexcitation of [Pyr1,4+][DCA–]. This was found to primarily reflect more efficient localization onto cavities rather than a slower geminate recombination rate.
Publication URL
Image
Publication Title
Photodetachment and Electron Dynamics in 1-Butyl-1-methyl-pyrrolidinium Dicyanamide
Publication Date