Citation
Yuriy V. Zatsikha, Tanner S. Blesener, Philip C. Goff, Andrew T. Healy, Rachel K. Swedin, David E. Herbert, Gregory T. Rohde, Kullapa Chanawanno, Christopher J. Ziegler , Rodion V. Belosludov, David A. Blank, and Victor N. Nemykin Journal of Physical Chemistry C, 2018, 122, 27893-27916.
Abstract
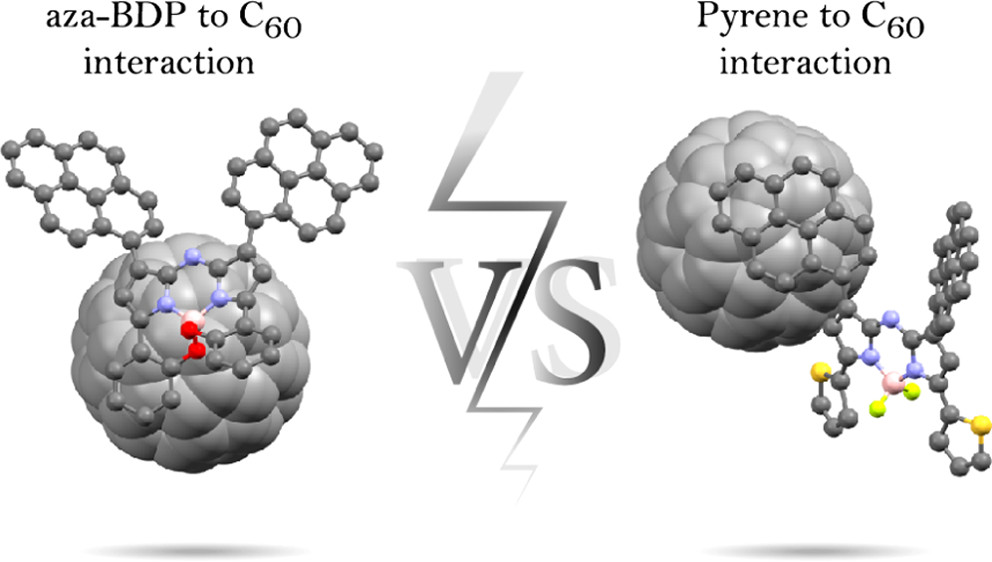
A series of 1,7-dipyrene-aza-BODIPY and 1,7-dipyrene-3,5-diferrocene-aza-BODIPY derivatives 5a–e with pyrene ligands covalently attached to the β-pyrrolic positions of the boron-azadipyrromethene (aza-BODIPY) core have been prepared and characterized by NMR, UV–vis, and steady-state fluorescence spectroscopy; high-resolution mass spectrometry; and X-ray crystallography. The redox processes of these donor–acceptor aza-BODIPY systems were investigated by electrochemistry (cyclic voltammetry and differential pulse voltammetry methods) and spectroelectrochemistry. The potential of the closely spaced 1,7-dipyrene fragments to promote formation of noncovalent π-complexes with nanocarbon materials (C60, C70, (6,5)-single-walled carbon nanotube, and graphene) was explored. UV–vis, steady-state fluorescent, and time-resolved transient absorption spectroscopy data indicated that the interaction between the new pyrene-aza-BODIPYs and C60 or C70 fullerenes in solution is weak, and time-resolved transient absorption spectroscopy provided no evidence of photoinduced electron transfer. X-ray crystallography on a binary solid of aza-BODIPYs 5b and C60 was indicative of a pyrene–pyrene rather than pyrene–C60 interaction motif, whereas fullerenes were found to form close contacts with the electron-rich B,O-chelated part of aza-BODIPY 5b. In contrast, pyrene–pyrene and pyrene–C60 but not aza-BODIPY–C60 interactions were observed in the crystal structure of aza-BODIPY 5d and C60. Density functional theory (DFT) and time-dependent DFT calculations were used to support conclusions based on experimental data and are suggestive of rather weak interaction energies between 1,7-dipyrene-aza-BODIPYs and nanocarbon materials. Direct comparison with an analogous control compound lacking the pyrene ligands demonstrated that the pyrene substituents were ineffective at promoting and directing complex formation with nanocarbon materials. A common measurement of complex formation, emission loss in the presence of a nanocarbon acceptor, was demonstrated to have an alternative explanation in these systems, and the general effectiveness of using pyrene ligands to build noncovalent complexes was drawn into question.
Publication URL
Image
Publication Title
1,7-Dipyrene-Containing Aza-BODIPYs: Are Pyrene Groups Effective as Ligands To Promote and Direct Complex Formation with Common Nanocarbon Materials?
Publication Date